O-Amino-p-Chlorophenol
Chemical Structure
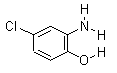
Product name: o-amino-p-chlorophenol
Other names: 4-chloro-2-aminophenol; p-chloro-o-aminophenol; o-amino-p-chlorophenol; 4CAP; 5-chloro-2-hydroxyaniline; 2-hydroxy-5-chloroaniline
molecular formula: C6H6ClNO
formula weight: 143.57
Numbering System
CAS No: 95-85-2
EINECS No: 202-458-9
Physical Data
Appearance: white or off-white crystalline powder.
Purity: ≥98.0%
Melting point: 140~142℃
Solubility: insoluble in water, solubility in water at 20°C <0.1 g/100 mL, soluble in ether, ethanol and chloroform.
Stability: stable when dry, easy to be oxidized and colored in humid air, flammable in case of open flame; high heat releases toxic chloride and nitrogen oxide gases.
Production Method
Used as a dye intermediate, and also used in the preparation of fluorescent whitening agent dye intermediates, and used in the production of fluorescent whitening agent DT.
Production Method
Using p-chlorophenol as a raw material, 2-nitro-p-chlorophenol can be made through nitration, and then reduced to make p-chloro-o-aminophenol.
(1) Production of 2-nitro-p-chlorophenol: Using p-chlorophenol as raw material, nitrification with nitric acid. Add the distilled p-chlorophenol slowly into the stirred tank with 30% nitric acid, keep the temperature at 25-30℃, stir for about 2 hours, add ice to cool to below 20℃, precipitate, filter, and wash the filter cake to Congo Red, the product 2-nitrop-chlorophenol is obtained.
(2) There are two methods for the reduction of 2-nitro-p-chlorophenol. One is to reduce with sodium disulfide. Firstly, 30% sodium hydroxide solution and sulfur powder are used to make sodium disulfide solution, and 2-nitro-p-phenol is added in proportion to react at 95-100°C, and the reaction is over. After hot filtration, the filtrate is neutralized with baking soda water, cooled to 20°C, filtered, and the filter cake is washed to neutrality to obtain the finished product 2-nitro-p-chlorophenol.
The second is the hydrogenation reduction method. In the presence of a nickel catalyst, the aqueous suspension of 2-nitro-p-chlorophenol is adjusted to pH=7 with sodium dihydrogen phosphate hydrate and sodium hydroxide aqueous solution at a hydrogen pressure of 4.05Mpa and Hydrogenation reduction at 60°C. After the reaction is complete, release the pressure, replace with nitrogen, heat to 95°C, adjust pH=10.7 with sodium hydroxide, add activated carbon and diatomaceous earth, stir vigorously, and filter. The filtrate was adjusted to pH=5.2 (20°C) with concentrated hydrochloric acid, cooled to 0°C, filtered, dried, and treated with sodium bisulfite. Repeat the operation four times, then distill at 2.67kpa, collect the fractions around 80°C, and dry them to obtain the product with a yield of 97.7%.
The Main Application
The main use of p-chloro-o-aminophenol is as a dye intermediate, for the preparation of acid mordant RH, acid complex violet 5RN and reactive dyes, etc., and also for the preparation of the raw material chlorzoxazone.
Packaging, Storage and Transportation
It is a dangerous chemical, and is packaged in 25kg iron drums, and the warehouse is ventilated, low-temperature and dry, and protected from direct sunlight. Keep away from fire heat sources, and store and transport separately from acids, oxidants, food additives, and oxidants.








